| References |
| Formal Name |
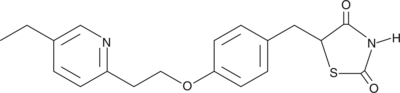
5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione |
| CAS Number |
111025-46-8 |
| Molecular Formula |
C19H20N2O3S |
| Formula Weight |
356.4 |
| Formulation |
A crystalline solid |
| Purity |
>98% |
| Stability |
1 year |
| Storage |
-20°C |
| Shipping |
Wet ice
in continental US; may vary elsewhere
|
Background Reading
Willson, T.M., Cobb, J.E., Cowan, D.J., et al. The structure-activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem 39 665-668 (1996).
Lehmann, J.M., Moore, L.B., Smith-Oliver, T.A., et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-acivated receptor γ (PPARγ). J Biol Chem 270 12953-12956 (1995).
Cantello, B.C.C., Cawthorne, M.A., Cottam, G.P., et al. [[ω-(Heterocyclylamino)alkoxy]benzyl]-2,4-thiazolidinediones as potent antihyperglycemic agents. J Med Chem 37 3977-3985 (1994).
Willson, T.M., Brown, P.J., Sternbach, D.D., et al. The PPARs: From orphan receptors to drug discovery. J Med Chem 43(4) 528-550 (2000).
Sakamoto, J., Kimura, H., Moriyama, S., et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun 278 704-711 (2000).
Show all 5
Hide all but first 3
| Size |
Global Purchasing |
| 1 mg |
|
| 5 mg |
|
| 10 mg |
|
| 50 mg |
|
Description
Thiazolidinediones (TZDs) are a group of structurally related PPARγ agonists with anti-diabetic actions in vivo.1,2 Rosiglitazone (BRL49653) is a prototypical TZD and has served as a reference compound for this class of PPARγ ligands.3 Pioglitazone is a closely related TZD which also selectively activates PPARγ-1. Pioglitazone is about one tenth as potent as rosiglitazone, with an EC50 of about 500-600 nM for both human and mouse PPARγ.4,5 In a transactivation assay using COS-1 cells transfected with full length human PPARα and RXRα, pioglitazone and rosiglitazone exhibit low level activation of PPARα at 1 µM and 5.4- and 4.2-fold activation, respectively, at a concentration of 10 µM.4
1
Willson, T.M., Cobb, J.E., Cowan, D.J., et al. The structure-activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem 39 665-668 (1996).
2
Cantello, B.C.C., Cawthorne, M.A., Cottam, G.P., et al. [[ω-(Heterocyclylamino)alkoxy]benzyl]-2,4-thiazolidinediones as potent antihyperglycemic agents. J Med Chem 37 3977-3985 (1994).
3
Lehmann, J.M., Moore, L.B., Smith-Oliver, T.A., et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-acivated receptor γ (PPARγ). J Biol Chem 270 12953-12956 (1995).
4
Sakamoto, J., Kimura, H., Moriyama, S., et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun 278 704-711 (2000).
5
Willson, T.M., Brown, P.J., Sternbach, D.D., et al. The PPARs: From orphan receptors to drug discovery. J Med Chem 43(4) 528-550 (2000).
|